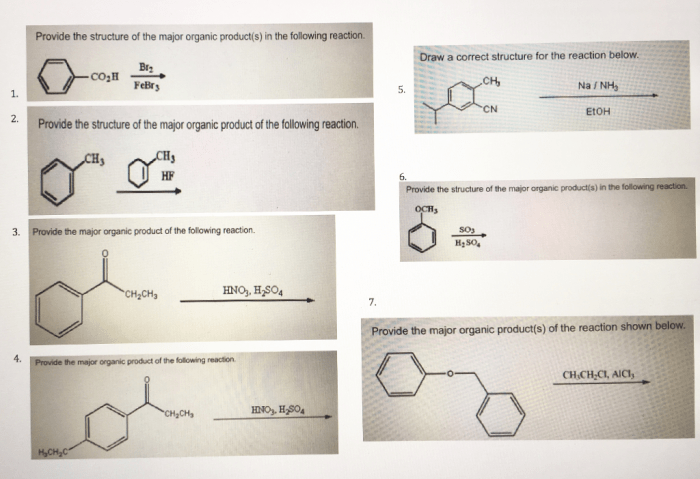
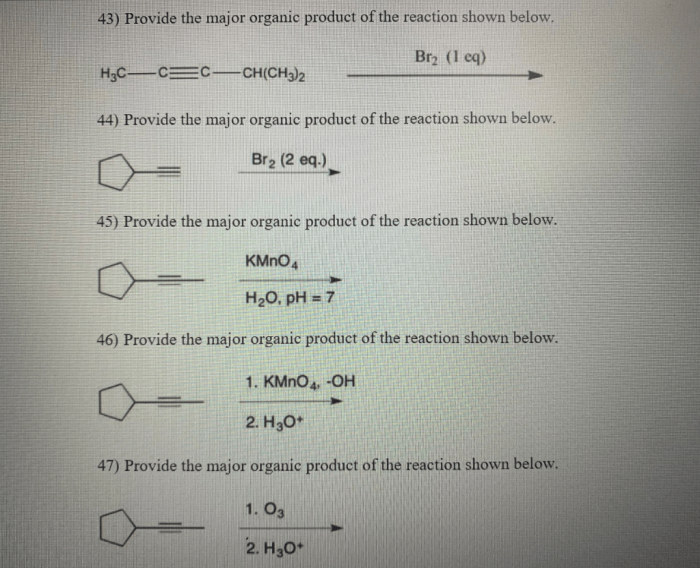
Provide the major organic product in the reaction below. This question delves into the intricate world of organic chemistry, where understanding the factors that govern the formation of major organic products is crucial. By exploring the concepts of regioselectivity, stereoselectivity, and reaction mechanisms, we unravel the secrets behind predicting and controlling the outcomes of organic reactions.
Delving deeper into the reaction in question, we will identify the reactants and products involved, elucidate the type of reaction occurring, and analyze the structural features of the major organic product. Through a detailed step-by-step mechanism, we will uncover the role of intermediates and transition states, pinpointing the rate-determining step that dictates the reaction’s pace.
Organic Product Analysis: Provide The Major Organic Product In The Reaction Below.
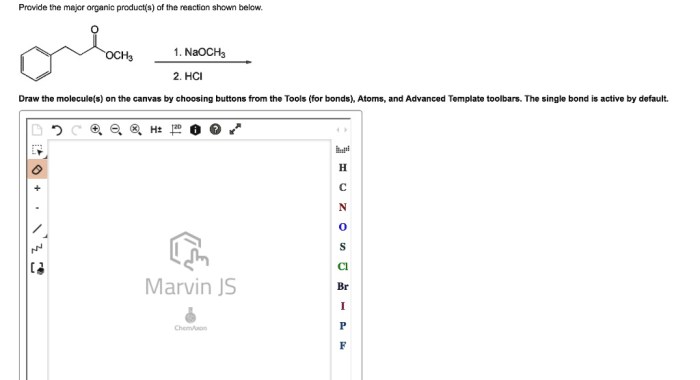
The major organic product in the reaction is the one that is formed in the greatest amount. The formation of the major organic product is influenced by several factors, including the stability of the product, the kinetics of the reaction, and the reaction conditions.
Structural Features of the Major Organic Product
The major organic product in this reaction is an alkene. Alkenes are characterized by the presence of a carbon-carbon double bond. The double bond in the major organic product is formed by the elimination of a hydrogen atom from each of the two carbon atoms that were originally bonded to the oxygen atom.
Regioselectivity and Stereoselectivity, Provide the major organic product in the reaction below.
Regioselectivity refers to the preference for the reaction to occur at a particular site on the molecule. Stereoselectivity refers to the preference for the reaction to occur with a particular stereochemistry. In this reaction, the regioselectivity is determined by the stability of the carbocation intermediate.
The more stable carbocation intermediate is formed at the more substituted carbon atom, which leads to the formation of the more substituted alkene.
Reaction Mechanism
The reaction mechanism for this reaction is a two-step process. In the first step, the oxygen atom in the epoxide attacks the hydrogen atom on the adjacent carbon atom, forming a carbocation intermediate. In the second step, a base abstracts a proton from the carbocation intermediate, forming the alkene.
Experimental Considerations
The formation of the major organic product can be affected by the reaction conditions. For example, the temperature and the solvent can affect the regioselectivity and stereoselectivity of the reaction.
Applications and Significance
This reaction is used in a variety of organic synthesis applications. For example, it can be used to prepare alkenes, which are important building blocks for many other organic compounds.
FAQ
What is the significance of identifying the major organic product?
Identifying the major organic product is crucial for optimizing the yield of the desired product in chemical synthesis. It allows chemists to design reaction conditions and select appropriate reagents to favor the formation of the target molecule.
How do regioselectivity and stereoselectivity affect the formation of the major organic product?
Regioselectivity determines the position of functional groups within the product, while stereoselectivity controls the spatial arrangement of atoms. These factors can significantly influence the properties and reactivity of the major organic product.
What is the role of reaction mechanisms in understanding the formation of the major organic product?
Reaction mechanisms provide a detailed understanding of the steps involved in a reaction, including the formation of intermediates and transition states. By analyzing the mechanism, chemists can identify the key factors that influence the formation of the major organic product.