Select all the elementary substances. – Elementary substances, the fundamental building blocks of the universe, form the foundation of chemistry and play a pivotal role in countless scientific disciplines. From the oxygen we breathe to the silicon in our computers, elementary substances are essential for life and technology.
This comprehensive guide delves into the fascinating world of elementary substances, exploring their unique properties, classification, applications, occurrence, and extraction methods. Prepare to embark on an enlightening journey into the realm of the elements.
Definition of Elementary Substances
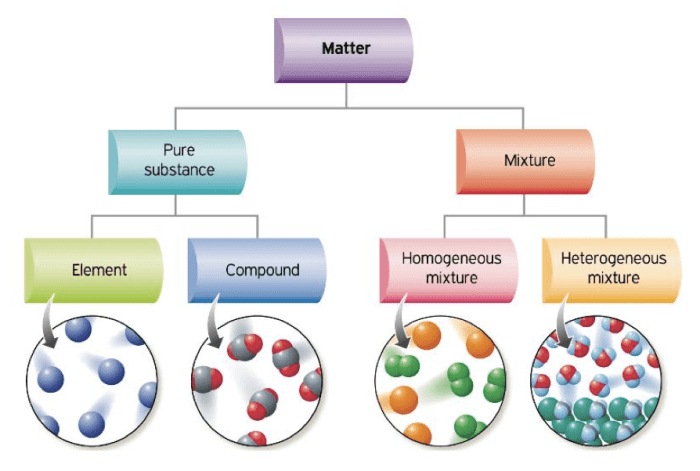
Elementary substances are the fundamental building blocks of matter and cannot be broken down into simpler substances by chemical means. They are characterized by their unique atomic structure and distinct properties.
Examples of elementary substances include:
- Hydrogen (H)
- Oxygen (O)
- Gold (Au)
- Iron (Fe)
- Chlorine (Cl)
Properties of Elementary Substances: Select All The Elementary Substances.
Elementary substances possess a wide range of physical and chemical properties that differentiate them from compounds and mixtures.
Reactivity:Some elements are highly reactive, such as sodium and chlorine, while others are relatively inert, like gold and platinum.
Conductivity:Metals are excellent conductors of electricity and heat, while non-metals are poor conductors.
Malleability:Metals are malleable, meaning they can be shaped or hammered into thin sheets without breaking.
Classification of Elementary Substances
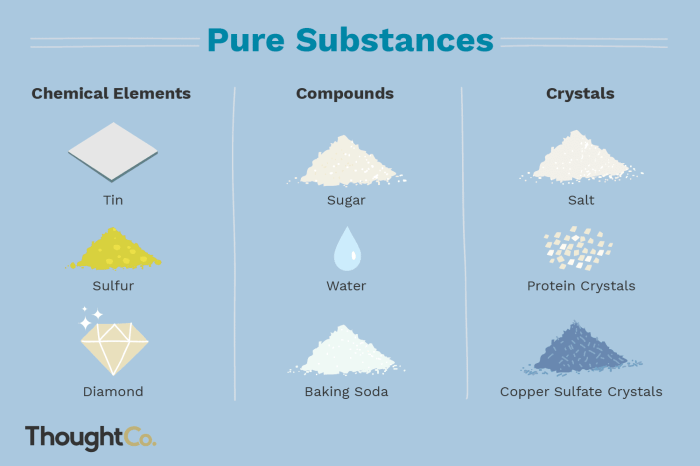
| Name | Symbol | Atomic Number | Group |
|---|---|---|---|
| Hydrogen | H | 1 | 1 |
| Oxygen | O | 8 | 16 |
| Gold | Au | 79 | 11 |
| Iron | Fe | 26 | 8 |
| Chlorine | Cl | 17 | 17 |
Applications of Elementary Substances
Elementary substances play a crucial role in various industries and applications:
- Electronics:Copper, silver, and gold are used in electrical wiring, circuits, and semiconductors.
- Construction:Iron and steel are essential for buildings, bridges, and other structures.
- Medicine:Iodine, fluorine, and oxygen are used in medical treatments and pharmaceuticals.
Occurrence of Elementary Substances

Elementary substances are found in various forms and locations in the Earth’s crust:
- Atmosphere:Nitrogen and oxygen make up the majority of the Earth’s atmosphere.
- Oceans:Sodium, chlorine, and magnesium are dissolved in seawater.
- Minerals and Rocks:Metals and other elements are found in minerals and rocks.
Extraction and Purification of Elementary Substances
Elementary substances are extracted and purified from their natural sources using various methods:
- Mining:Ores containing valuable metals are mined and processed to extract the desired element.
- Electrolysis:Electrolysis is used to extract metals from their molten salts or solutions.
- Distillation:Distillation is used to separate volatile elements from their impurities.
Top FAQs
What are the key characteristics of elementary substances?
Elementary substances are pure substances that cannot be broken down into simpler substances by chemical means. They are characterized by their unique atomic structure, chemical properties, and physical properties.
How are elementary substances classified?
Elementary substances are classified into metals, non-metals, and metalloids based on their properties. Metals are typically shiny, malleable, and good conductors of heat and electricity. Non-metals are typically dull, brittle, and poor conductors of heat and electricity. Metalloids exhibit properties of both metals and non-metals.
What are some common applications of elementary substances?
Elementary substances have a wide range of applications in various industries, including electronics, construction, medicine, and energy. For example, copper is used in electrical wiring, iron is used in steel production, and oxygen is used in medical treatments.